
Working with CRM to move Malaysia forward
The Malaysian government has committed funding to improve the healthcare systems in order to further support clinical research. Clinical Research Malaysia (CRM) is a non-profit organisation &# […]
了解更多
The Malaysian government has committed funding to improve the healthcare systems in order to further support clinical research. Clinical Research Malaysia (CRM) is a non-profit organisation &# […]
了解更多
乔治临床( George Clinical ) (GC) conducted a brief survey on sponsors who are currently not a customer of GC. 30 companies were surveyed by GC’s Business Development Managers across six cou […]
了解更多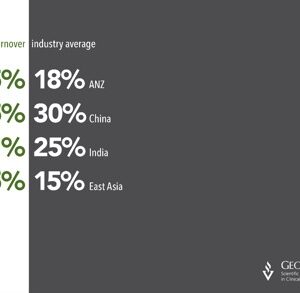
The cost of employee turnover in a Clinical Research Organisation (CRO) is high, as it weakens the relationship with the project sponsor. Research shows that in 2012, the CRO industry in the U […]
了解更多In a surprise move, the State Council in China has recently released a directive announcing its view on reforming the drug and medical device review and approval process in China. The State Co […]
了解更多乔治临床( George Clinical ) in China recently celebrated its second anniversary as an independent entity in China. Although 乔治临床( George Clinical ) has been conducting Phase 11 to Phase IV studies […]
了解更多乔治临床( George Clinical ) (GC) is at the heart of developing Asia Clinical Research Professionals. This summer, our Clinical and Regulatory Manager, East Asia volunteered as a trainer for Asia T […]
了解更多
The George Institute was recently profiled in The Lancet; revered as one of the most prestigious, peer reviewed medical journals in the world. The Lancet Vol. 385 April 18, 2015 The feature ar […]
了解更多
ARCS is Australia’s primary professional development association for people working in the development of therapeutic goods. At the annual scientific congress held in Sydney recently, Dr Maria […]
了解更多