
The Study
乔治临床( George Clinical ) (GC) collaborated with a mid-sized global pharmaceutical company to conduct a randomized trial comparing pain relief medication for patients with moderate to severe chronic cancer pain.
乔治临床( George Clinical ) services
GC was responsible for project management (PM) and monitoring across 26 sites in China. Study sites were located in Beijing, Changchun, Dalian, Fuzhou, Guangzhou, Hangzhou, Harbin, Jinan, Nanjing, Shanghai, Shenyang, Shijiazhuang, Tianjin, Wuhan, Xiamen, Xi’an and Xinxiang.
Challenges
The team encountered several challenges that caused a delay to the project commencing. Originally, the protocol excluded patients undergoing chemotherapy and with a biomarker commonly found in advanced cancer patients. Also, the original site list provided to the GC team included sites that were insufficiently resourced for the trial.
Expert Solutions
The GC Project Manager (PM) liaised closely with investigators and sponsors to update the protocol in order for it to be more realistic. Unsuitable sites were quickly replaced with sites identified through the Principal Investigator’s network, GC and the sponsor’s database. Recognizing that the project timelines had become challenging, the project manager prepared efficient work plans and devised additional strategies to reach recruitment levels. These strategies consisted of a monthly newsletter that communicated site recruitment experiences, small rewards that incentivised top enrolling sites and establishing interim project forums. GC fastidiously maintained communication with the sponsor in order to identify any issues and provide solutions early in the project.
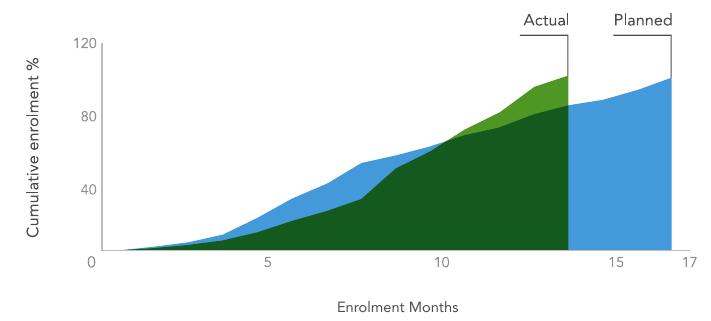
GC’s project team, provided exceptional metrics through preparation such as early risk identification, efficient work plans, activities and positive teamwork. As a result, the first patient in was achieved within 3 months of contract signature, the first 50% of patients within 7 months, and the remaining 50% within 8 months. In total, the project team achieved the last patient 3 months ahead of schedule, despite initial challenges.
Chinese Registration Study Enrolment Performance
About 乔治临床( George Clinical )
乔治临床( George Clinical ) is a leading clinical research organisation in the Asia-Pacific region with over 260 staff operating in 15 countires. 乔治临床( George Clinical ) provides a full range of trial management services to pharmaceutical and biotech customers, for both registration and post marketing trials. Our parent organization, The George Institute, is a leader in chronic disease research with a global network of experts. 乔治临床( George Clinical ) combines this scientific and clinical leadership from the Institute with world class trial delivery capability to create a distinctive service. Our internationally recognized scientific leadership allows 乔治临床( George Clinical ) to provide excellence from design to delivery. Gain access to Asia.


